Hybridization as a mechanism
of gene flow and speciation in plants (A case study
of Quercus in the Four Corners region)


|
Q. gambelii var. bonina
Q. turbinella
Q. gambelii X turbinella
Senior Seminar Documents
Undergraduate Student Research, Scholarly and Creative Activities grant program
Due November 2
Techniques
Updated DNA extraction protocol (using Qiagen kit)
Updated ISSR and agarose gel protocol
4-corners Oak collection locations
Specimen Mapping and Ecological Niche Analysis
Research Presentation
The Biology Department Senior Seminar Poster Session will be April 6.
Biology Department guidelines for poster preparation
Photos of Quercus hybrids across the Four Corners region Note: These images have not been resized for easy and quick web viewing but rather for good resolution in your posters.
Directions for poster preparations and sample posters - Provided by Dr. Cynthia Dott
Creating a poster in PowerPoint
|
The genus Quercus is well-known for having low barriers to gene flow among different species. This hybridization can lead, in areas where multiple taxa coincide, to the production of hybrid individuals. These individuals often show intermediary morphological characteristics to the two parental taxa. In areas of widespread gene exchange and introgression this can lead to a wide diversity of plant forms across the landscape. The prevalence of hybridization in groups such as Quercus has led to a difficulty in delimiting and even defining what a species is. While it can be relatively straightforward to identify hybrid individuals once the characters of the parental taxa are well-known, this morphological determination of the prevalence of hybridization can be misleading and not indicate the full extent of the hybrid occurrence. Genetic data are thus key to determine the relative genomic contribution from various parental forms.
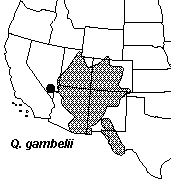
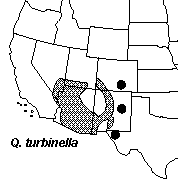
The Four Corners area of Arizona, Colorado, New Mexico, and Utah is principally dominated by two species of Quercus, Q. gambelii and Q. turbinella.
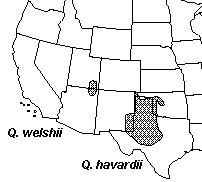
Across the region various hybrid populations and individuals are known which have been commonly referred to as Q. undulata. Quercus undulata is a variable complex of hybrid derived forms extending across the southwestern United States and influenced by numerous parental taxa (Tucker, 1961; Tucker et al., 1961). The Four Corners area also shows a few more geographically restricted forms of Quercus. Plants from the Navajo Basin of Utah and Arizona have normally been treated as a distinct variety of Q. havardii a species better known from NW Texas and adjacent New Mexico and Oklahoma. This taxon, Q. havardii var. tuckerii, has been hypothesized to represent a variable hybrid form and may not be allied to Q. havardii. Following this rational it was recently transferred to a descretely named taxon, Q. welshii. An additional discrete variety of Q. gambelii has also recently been described from SE Utah, Q. gambelii var. bonina.
Range maps © Flora of North America Seminar Goals The goal of this seminar is to lean how natural hybridization can act as a means of speciation and evaluate patterns of hybridization in a group in which hybridization is known to be an important factor in evolution. To do this we will be studying Quercus across the Four Corners region to evaluate the extent and patterns of hybridization using a variety of organismic biology tools. While we will all be studying the same system differing groups of students will focus on the completion of different parts of the study. 1. Mapping of distribution of parental species using herbarium specimen databases and GIS. 2. Morphometric analysis of leaf form among various taxa. 3. Genetic analysis using Inter-simple Sequence Repeat markers (ISSR) Calendar for Student Presentations
Papers for Student Presentations Each title links to a PDF of the article.
Borazan, A. and M. T. Babaç. 2003. Morphometric leaf variation in oaks (Quercus) of Bolu, Turkey. Ann. Bot. Fennici 40: 233-242.
González-Rodríguez, A, D. M. Arias, S. Valencia, and K. Oyama. 2004. Morphological and RAPD analysis of hybridization between Quercus affinis and Q. laurina (Fagaceae), Two Mexican red oaks. American Journal of Botany 91: 401-409.
González-Rodríguez, A, J. F. Bain, J.L. Golden, and K. Oyama. 2004. Chloroplast DNA variation in the Quercus affinis–Q. laurina complex in Mexico: geographical structure and associations with nuclear and morphological variation. Molecular Ecology 13: 3467-3476.
Hokanson, S.C., J.G. Isebrands, R.J. Jensen, and J.F. Hancock, 1993. Isozyme variation in oaks of the Apostle Islands in Wisconsin: genetic structure and levels of inbreeding in Quercus rubra and Q. ellipsoidalis (Fagaceae). American Journal of Botany 80: 1349-1357.
Howard, D. J. R. W. Preszler, J. Williams, S. Fenchel, and W. J. Boecklen. 1997. How discrete are oak species? Insights from a hybrid zone between Quercus grisea and Quercus gambelli. Evolution 51: 747-755.
Jensen, R. J., S. C. Hokanson, J. G. Isebrands, and J. F. Hancock. 1993. Morphometric variation in oaks of the Apostle Islands in Wisconsin: Evidence for hybridization between Quercus rubra and Quercus ellipsoidalis (Fagaceae). American Journal of Botany 80: 1358-1366.
Kothera, L., S. M. Ward, S. E. Carney.
2007.
Assessing the threat from hybridization to the rare endemic
Physaria bellii Mulligan (Brassicaceae). Biological
Conservation 140: 110-118. Lau, C. P. Y., L. Ramsden, and R. M. K. Saunders. 2005. Hybrid origin of "Bauhinia blakeana" (Leguminosae: Caesalpinioideae), inferred using morphological, reproductive, and molecular data. American Journal of Botany 92: 525-533.
Muir, G. and C. Schlötterer. 2005. Evidence for shared ancestral polymorphism rather than recurrent gene flow at microsatellite loci differentiating two hybridizing oaks (Quercus spp.). Molecular Ecology 14: 459-561.
Rubio de Casas, R., E. Cano, L. Balaguer, E. Pérez-Corona, E. Manrique, C. García-Verdugo, P. Vargas. 2007. Taxonomic identity of Quercus coccifera L. in the Iberian Peninsula is maintained in spite of widespread hybridisation, as revealed by morphological, ISSR and ITS sequence data. Flora 202: 488-499.
Tovar-Sánchez, E and K. Oyama. 2004. Natural hybridization and hybrid zones between Quercus crassifolia and Quercus crassipes (Fagaceae) in Mexico: Morphological and molecular evidence. American Journal of Botany 91: 1352-1363.
Valbuena-Carabaña, M, S. C. González-Martínez, V.L. Sork, C. Collada, A. Soto, P.G. Goicoechea, and L. Gil. 2005. Gene flow and hybridisation in a mixed oak forest (Quercus pyrenaica Willd. and Quercus petraea (Matts.) Liebl.) in central Spain. Heredity 95: 457-465.
Zalapa, J. E., J. Brunet and R. P. Guries. 2009. Patterns of hybridization and introgression between invasive Ulmus pumila (Ulmaceae) and native U. rubra. American Journal of Botany 96: 1116-1128.
Important Literature (Useful background reading for proposal preparation) The following literature should serve as a base for your understanding of natural hybridization in Quercus and the specifics of the Four Corners species of Quercus.
Anderson, E. and G. L. Stebbins, Jr. 1954. Hybridization as an evolutionary stimulus. Evolution 8: 378-388.
Baack, E. J. and L. H. Rieseberg. 2007.
A genomic view of introgression and hybrid speciation.
Current Opinion in Genetics & Development 2007. 17: 513–518. Cristofolini, G. and S. Crema. 2005. A morphometric study of the Quercus crenata species complex (Fagaceae). Botanica Helvetica 115: 155-167.
Mallet, J. 2007. Hybrid speciation. Nature 446: 279-283.
Petit, R., C. Bodenes, A. Ducousso, G. Roussel, and A. Kremer. 2003. Hybridization as a mechanism of invasion in oaks. New Phytologist 161: 151-164.
Rieseberg, L. H. 1995. The Role of Hybridization in Evolution: Old Wine in New Skins. American Journal of Botany 82: 944-953.
Soltis,
P. S. and D. E. Soltis. 2009. The role of hybridization in plant
speciation. Annual Review of Plant Biology 60: 561-588. Tucker, J. M. 1961. Studies in the Quercus undulata complex. I. A preliminary statement. American Journal of Botany 48: 202-208.
Tucker, J. M. 1963. Studies in the Quercus undulata complex. III. The contribution of Q. arizonica. American Journal of Botany 50: 699-708.
Tucker, J. M. 1970. Studies in the Quercus undulata complex. IV. The contribution of Quercus havardii. American Journal of Botany 57: 71-84.
Tucker, J. M. 1971. Studies in the Quercus undulata. V. The type of Quercus undulata. American Journal of Botany 58: 329-341.
Tucker, J. M., W. P. Cottam, and R. Drobnick. 1961. Studies in the Quercus undulata complex. II. The contribution of Quercus turbinella. American Journal of Botany 48: 329-339.
Van Valen, L. 1976. Ecological species, multispecies, and oaks. Taxon 25: 233-239.
Welsh, S. L. 1986. Quercus (Fagaceae) in the Utah flora. Great Basin Naturalist 46: 107-111.
Whittemore, A. T. and B. A. Schaal. 1991. Interspecific gene flow in sympatric oaks. Proceedings of the National Academy of Sciences, USA. 88: 2540-2544.
Last Updated 26 March 2010
|